How Many Valence Electrons in Rubidium
Herewith Rubidium Rb has 1 valence electron. Therefore the number of electrons in neutral atom of Rubidium is 37.

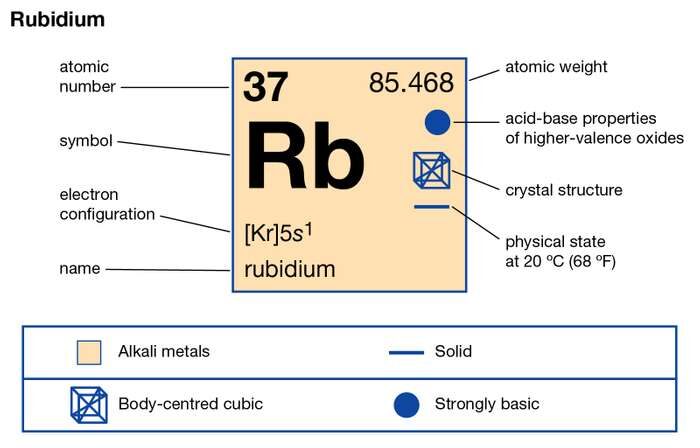
Rb Rubidium Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus.
. For main group elements ie s-block and p-block elements the valence. 37 electrons Rubidium Atomic and Orbital Properties Rubidium atoms have 37 electrons and the electronic shell structure is 2 8 18 8 1 with Atomic Term Symbol Quantum Numbers 2S12. Valence Electrons in Rubidium Rb Facts Color Discovery.
Rubidium has a total of 37 electrons illustrated in the elements electron configuration of 1s2 2s2p6 3s2p6d10 4s2p6 5s1. This electron configuration shows that the last shell of rubidium has only an electron. What is the symbol for this element.
How many valence electrons does an atom of rubidium Rb atomic number 37 have. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. How many valence electrons does Rubidium.
It means that Rubidium atom has 37 protons and 37 electrons. How many electrons are in the last shell of rubidium. Rubidium has one valence electron which is located in the s-orbital of the atoms fifth energy level.
Rubidium has a total of 37 electrons illustrated in the elements electron configuration of 1s2 2s2p6 3s2p6d10 4s2p6 5s1. Rb valency is not zero like noble gas as. Therefore the valence electrons of rubidium are one.
Rubidium is in Group I of the periodic table hence it has 1 valence electron. Rubidium is represented by the chemical symbol Rb on the periodic table of elements. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom.
The elements that form bonds by donating electrons are called cations. Rubidium has a total of 37 electrons illustrated in the elements electron configuration of 1s2 2s2p6 3s2p6d10 4s2p6 5s1. Answer the questions below for an element that has the electron configuration 1s2 2s2 2p6 3s2 3p6 4s1.
An atom with three valence electrons I think it is B. Rubidium has one valence electron which is located in the s-orbital of the atoms fifth energy level. The electrons in the last shell is equal to rubidium valence electrons.
Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom. Therefore the number of electrons in neutral atom of Rubidium is 37. The elements that have 1 2 or 3 electrons in the last shell donate the electrons in the last shell during bond formation.
We can find how many valence electrons present in the last shell of Rubidium by writing electron configuration of Rubidium. As there is only 1 electron in Rubidiums valence shell Rubidiums valency is 1. Up to 24 cash back The reason for this is rubidium is a much larger atom than sodium since it has 5 energy.
6 rows Thus rubidium ion Rb has eight valence electrons. Rubidium has one valence electron which is located in the s-orbital of the atoms fifth energy level. Rubidium is a chemical element with symbol Rb and atomic number 37.
Rubidium has one valence electron which is located in the s-orbital of the atoms fifth energy level. AOne BFive CSix D37 is it a pls help me ms sue or damon or anybody. Classified as a n alkali metal Rubidium is a solid at room temperature.
An atom with two valence electrons D.

Rubidium Valence Electrons Rubidium Valency Rb Dot Diagram

Valency Of Rubidium How Many Valence Electrons Does Rubidium Rb Have
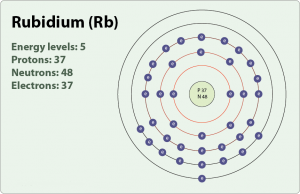
How Many Valence Electrons Does Rubidium Have Archives Dynamic Periodic Table Of Elements And Chemistry

Comments
Post a Comment